Abstract
We recently reported on a syndrome in humans caused by VPS4A mutations characterized by dyserythropoiesis, hemolytic anemia, and neurodevelopmental delay. VPS4A is an ATPase that participates with the Endosomal Sorting Complex Required for Transport (ESCRT)-III complex in a variety of cellular processes including the abscission step of cytokinesis and endo-lysosomal trafficking. De novo dominant-negative mutations in the ATPase domain of VPS4A are associated with erythroblast cytokinesis failure and altered trafficking of the transferrin receptor (TfR1/CD71) consistent with known roles for ESCRT-III in cell division and endosomal transport. However, the mechanisms by which ESCRT-III dysfunction impairs erythropoiesis remain unclear.
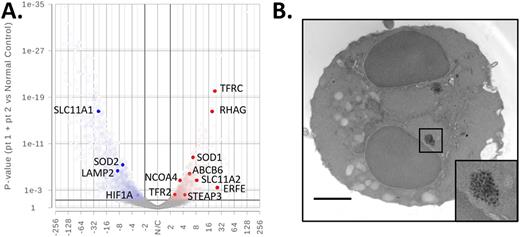
In addition to erythroid dysplasia in the bone marrow, patients with dominant-negative VPS4A mutations have hypochromic reticulocytes exhibiting high levels of TfR1 expression and mature RBCs which aberrantly retained TfR1 on the membrane. We further evaluated the reticulocytes from two patients with heterozygous VPS4A G203E mutations, one of the two previously described dominant-negative mutations causing this syndrome. In labeled-transferrin uptake assays, patient reticulocytes endocytosed less transferrin than control reticulocytes despite having increased TfR1 on their surface. RNA-sequencing analysis of CD71+ reticulocytes from patients and normal controls revealed dysregulated expression of genes involved in iron transport and homeostasis including upregulation of STEAP3 metalloreductase and Divalent Metal Transporter 1, DMT1 (SLC11A2), involved in export of iron from the endosome to the cytoplasm (4.4-fold, p<0.01 and 8.4-fold, p<0.001, respectively) (Figure 1A). As expected, expression of TFRC, encoding TfR1/CD71, was markedly increased (22-fold, p<0.001). By electron microscopy (EM), we observed evidence of iron overload within patient erythroblasts in the form of electron dense ferritin granules (Figure 1B); EM images also revealed irregular endolysosomal compartments. Adult mice with erythroid-specific expression of a dominant-negative VPS4 mutant (EporCre-Vps4bE235Q) develop anemia. Vps4bE235Q transgenic bone marrow phenocopies human disease containing binucleated erythroblasts and cytoplasmic bridges connecting erythroblasts, indicating this mouse model could be used to further investigate the pathogenic mechanisms of VPS4 mutations on erythropoiesis. Taken together, our studies on human and murine erythropoiesis provide the first evidence that dominant-negative VPS4A mutations, causing erythroblast cytokinesis failure and binucleated erythroblasts, also disrupt iron uptake and trafficking through the endo-lysosomal pathway of terminal erythropoietic cells.
Disclosures
Cancelas:Hemanext LLC: Consultancy; TerumoBCT: Consultancy; Vascular Solutions/Teleflex Inc: Consultancy; Cytosorbents Inc: Consultancy; Westat Inc: Research Funding; Cytosorbents Inc.: Research Funding; Fresenius-Kabi: Research Funding; TerumoBCT: Research Funding; Becton-Dickinson: Research Funding; Platefuse Inc: Other: Co-Ownership; Preservation Bio Inc: Other: Co-Ownership. Kalfa:Forma Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Agios Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal